We now investigate the effect of including the induction energy for the SCM LJ
parameters. We find that apart from increasing the expense of the calculations, the
PES's remain qualitatively unchanged to the extent that most minima look the same,
and for BzAr with ![]() the lowest-lying minima are effectively unchanged.
Fig. 5 shows the contribution of the induction energy for a selection
of minima and the results are easy to interpret. The size of the induction energy
contribution is mostly attributable to the location of the Ar atoms relative to the
benzene quadrupole. An Ar atom above the
the lowest-lying minima are effectively unchanged.
Fig. 5 shows the contribution of the induction energy for a selection
of minima and the results are easy to interpret. The size of the induction energy
contribution is mostly attributable to the location of the Ar atoms relative to the
benzene quadrupole. An Ar atom above the ![]() axis of the benzene molecule will be
most stabilised by the inclusion of the induction energy, whereas an atom lying in
the plane of the molecule will be least stabilised. This follows from the properties of the
quadrupole moment on the benzene molecule. The best illustration of this is
for the simplest system, BzAr
axis of the benzene molecule will be
most stabilised by the inclusion of the induction energy, whereas an atom lying in
the plane of the molecule will be least stabilised. This follows from the properties of the
quadrupole moment on the benzene molecule. The best illustration of this is
for the simplest system, BzAr ![]() , for which there is just one rearrangement
mechanism, involving the
, for which there is just one rearrangement
mechanism, involving the ![]() minimum and only one transition state,
(0|1|0), not counting permutational isomers. The minimum has
minimum and only one transition state,
(0|1|0), not counting permutational isomers. The minimum has
![]() symmetry, the Ar atom lying above the centre of the ring, and the transition
state has
symmetry, the Ar atom lying above the centre of the ring, and the transition
state has ![]() symmetry with the Ar atom in the plane of the ring, equidistant
from two adjacent H atoms. For the minimum the contribution of the induction energy
to the total energy is 6.76%; for the transition state the contribution is only
0.680%.
symmetry with the Ar atom in the plane of the ring, equidistant
from two adjacent H atoms. For the minimum the contribution of the induction energy
to the total energy is 6.76%; for the transition state the contribution is only
0.680%.
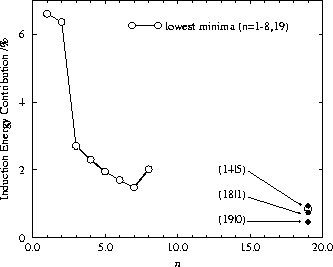
We are now in a position to explain the results in Fig. 5. For BzAr ![]() ,
the induction energy contribution is also relatively high because the second Ar atom
is situated directly below the centre of the benzene molecule i.e. diametrically
opposite the first Ar. The lowest minima found for BzAr
,
the induction energy contribution is also relatively high because the second Ar atom
is situated directly below the centre of the benzene molecule i.e. diametrically
opposite the first Ar. The lowest minima found for BzAr ![]() to BzAr
to BzAr ![]() have smaller
contributions as the positions of the Ar atoms are no longer optimal with respect to
the molecular quadrupole moment, especially as they are all one-sided. The
stabilisation due to Ar-Ar dispersion interactions is proportionally greater than
the change in the induction energy. At BzAr
have smaller
contributions as the positions of the Ar atoms are no longer optimal with respect to
the molecular quadrupole moment, especially as they are all one-sided. The
stabilisation due to Ar-Ar dispersion interactions is proportionally greater than
the change in the induction energy. At BzAr ![]() we see an increase again -- this is
due to the global minimum being two-sided with one Ar atom on the
we see an increase again -- this is
due to the global minimum being two-sided with one Ar atom on the ![]() axis below
the benzene molecule, which is again an optimal position for stabilisation via the
induction contribution.
axis below
the benzene molecule, which is again an optimal position for stabilisation via the
induction contribution.
For our BzAr ![]() lowest minimum the contribution of the induction energy is only
0.849% because many of the Ar atoms are far from the
lowest minimum the contribution of the induction energy is only
0.849% because many of the Ar atoms are far from the ![]() axis of the benzene
molecule. Our lowest lying (19|0) minimum has a 0.462% contribution, which is
smaller because there is no Ar atom in an optimal position below the benzene
molecule. We also found an (18|1) type structure with a contribution of
0.745%, and more fully `solvated'
(14|5) minimum with a contribution of 0.940% -- this structure has a more
favourable arrangement of Ar atoms with respect to the molecular quadrupole moment.
axis of the benzene
molecule. Our lowest lying (19|0) minimum has a 0.462% contribution, which is
smaller because there is no Ar atom in an optimal position below the benzene
molecule. We also found an (18|1) type structure with a contribution of
0.745%, and more fully `solvated'
(14|5) minimum with a contribution of 0.940% -- this structure has a more
favourable arrangement of Ar atoms with respect to the molecular quadrupole moment.
For BzAr ![]() the variation of the contribution of the induction energy with
respect to the structure can be
easily rationalized and it is always small
the variation of the contribution of the induction energy with
respect to the structure can be
easily rationalized and it is always small ![]() . We use this argument to
justify our neglect of the induction energy in subsequent Monte Carlo and Molecular
Dynamics simulations, which run many times faster in this approximation.
. We use this argument to
justify our neglect of the induction energy in subsequent Monte Carlo and Molecular
Dynamics simulations, which run many times faster in this approximation.