The PES of BzAr ![]() is very simple, there being only one minimum and one transition state. We use
two approaches to estimate the rate at which the Ar atom crosses the plane of the benzene molecule,
namely MD and the methods developed by Rice and Ramsperger, and
Kassel [50] (RRK).
is very simple, there being only one minimum and one transition state. We use
two approaches to estimate the rate at which the Ar atom crosses the plane of the benzene molecule,
namely MD and the methods developed by Rice and Ramsperger, and
Kassel [50] (RRK).
For MD, we use 43ns trajectories with total energy between the energy of the transition state and the
dissociation energy. Our MD results for BzAr ![]() use the SCM parameters only but here we
present, for comparison, those obtained with OBJ as well. At regular time intervals we analyse the Cartesian
coordinates for all atoms in the system to determine which side of the ring the Ar
atom is on and hence count the number of times the Ar atom crosses the ring. This leads
directly to the side-crossing rate which converges well on the given time-scale.
use the SCM parameters only but here we
present, for comparison, those obtained with OBJ as well. At regular time intervals we analyse the Cartesian
coordinates for all atoms in the system to determine which side of the ring the Ar
atom is on and hence count the number of times the Ar atom crosses the ring. This leads
directly to the side-crossing rate which converges well on the given time-scale.
RRK theory assumes that the kinetic energy is accessible to all the vibrational normal modes in equal proportion. It can then be shown that the rate constant as a function of energy is
where ![]() is the order of the point group; {
is the order of the point group; { ![]() }, the normal mode frequencies; E, the
energy measured from zero at the minimum;
}, the normal mode frequencies; E, the
energy measured from zero at the minimum; ![]() , the energy of the transition state and s is
the number of vibrational degrees of freedom in the system, i.e. 3 for BzAr
, the energy of the transition state and s is
the number of vibrational degrees of freedom in the system, i.e. 3 for BzAr ![]() with the benzene
molecule considered rigid.
The unlabelled quantities refer to the minimum; those labelled with
with the benzene
molecule considered rigid.
The unlabelled quantities refer to the minimum; those labelled with ![]() ,
to the transition
state.
,
to the transition
state.
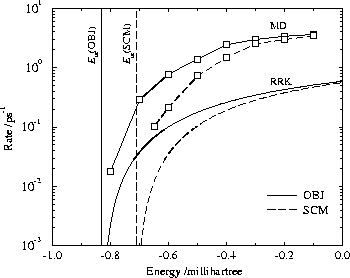
The results are plotted in Fig. 15 and show that the RRK results are always within one order of magnitude of those calculated from MD. The RRK results are translated such that the energy is zero at dissociation. Both the behaviour at the transition state energies and close to dissociation are in quite good agreement.
We present these results for comparison with BzAr ![]() for which we estimate barriers
for one-sided to two-sided transitions from the MD trajectories. Calculating
ring-crossing rates may not be as useful, as we have the possibility of multiple bridging
atoms, and
using RRK theory is tedious because of the number of transition states we would have to consider.
for which we estimate barriers
for one-sided to two-sided transitions from the MD trajectories. Calculating
ring-crossing rates may not be as useful, as we have the possibility of multiple bridging
atoms, and
using RRK theory is tedious because of the number of transition states we would have to consider.